The FDA encourages the digital therapy innovation unicorn VS business alliance, who will highlight the encirclement?
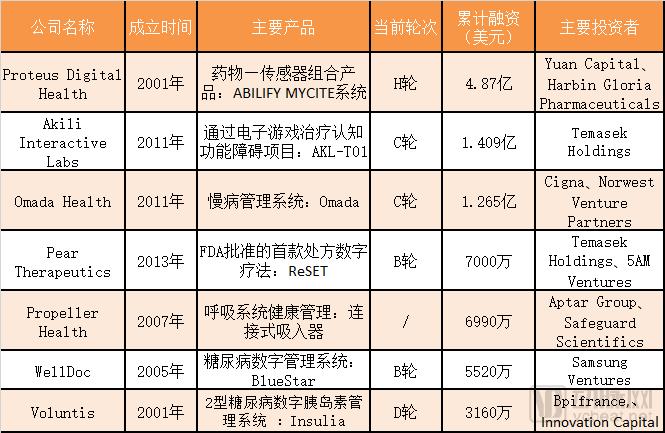
Recently, PEAR Therapeutics' product ReSET became the first FDA-approved digital prescription product to be used in the adjuvant treatment of drug abuse disorders such as stimulants, marijuana, cocaine or alcohol to help patients control drug addiction. In recent years, with the emergence of digital innovators and regulators adjusting their auditing frameworks, digital therapies have developed rapidly and become emerging markets with great potential. Pharmaceutical companies are also investing heavily in digital health start-ups, and software-based digital therapies have emerged on traditional drug-led pharmaceutical manufacturers' production lines. The Arterial Network takes stock of the major competitors in this field. This article includes the following: 1. Comprehensive analysis of digital therapy: FDA is open; 2. The main competitive landscape: Unicorn VS Enterprise Alliance; 3. Development potential: As an auxiliary treatment, it brings opportunities for pharmaceutical companies. Comprehensive analysis of digital therapy: FDA is open-minded In the past decade, the digital health field has developed very rapidly, and researchers have used advanced technology or equipment to improve clinical treatment, resulting in two distinct types of digital therapies. The first is a treatment that extends the value of traditional medicines, for example, by providing complementary medication management and personalized treatment recommendations through complementary software to help patients manage their condition, including the timing and dose of medication. The second is a treatment that replaces traditional medicines, such as delivering sensory stimuli through a tablet to treat insomnia or depression. Digital Therapeutics Alliance, a group of industry stakeholders, defines digital therapy (DTx) as: “Digital therapy provides patients with evidence-based treatments that are driven by high-quality software programs to prevent, manage, or treat a disease. They can be used alone or in combination with drugs, equipment, or other therapies. To optimize treatment outcomes and improve patient health. Digital therapy products combine advanced technologies related to design, clinical trials, usability, and data security. Regulators need to review and approve these products to address product claims related to risks, efficacy, and intended use. Digital therapies provide patients, health care providers, and payers with a variety of smart tools to deal with disease through high-quality, safe, and effective data-driven interventions. †In particular, digital therapy is often aimed at problems that are currently not well addressed by the medical system, such as chronic or neurological diseases. In addition, this therapy is usually cheaper than traditional therapies by reducing the time required for clinicians. At the same time, more and more evidence shows their clinical value. For example, the US Food and Drug Administration (FDA) recently approved a mobile app that helps treat alcohol, marijuana, and cocaine addiction, and clinical trials show that 40% of patients using the app Addiction or dependence was quit within three months, and only 17.6% of patients who received standard therapy alone quit addiction or dependence. Currently, hundreds of millions of dollars are invested in digital health technology, and there are more than 318,000 related applications on the market. In this process, regulators play an important role, they need to conduct a rigorous review and evaluation of digital therapy products, telling consumers and medical professionals what is safe, effective, therapeutic and clinical. We can see that for digital therapy products, the FDA is open and encourages innovation. FDA Director Scott Gottlieb once said, "Under the protection of patients, the FDA is expanding the development opportunities for digital healthcare and actively developing new regulatory frameworks to review related products in new ways." For example, its affiliated device and radiation health center created the digital health department, a move that suggests that the FDA is beginning to think about how to evaluate a software or application, and the criteria must be different from traditional medicines and medical devices to accommodate digital health technologies. Uniqueness and its different business innovation cycles. Pear Therapeutics' ReSET became the first FDA-approved prescription digital therapy; the ABILIFY MYCITE system, developed by Proteus Digital Health and Otsuka Pharmaceutical, was approved by the FDA for New Drug Application (NDA), becoming the first approved drug-sensor combination; Akili A multicenter trial was recently completed and publicly requested for FDA approval. These milestones show that digital therapy is gaining recognition and support from regulators and is an important area of ​​the digital health market. In addition to recent approvals, the FDA appears to be paving the way for more digital prescription products. The FDA's Pre-Cert for Software Pilot Program allows companies to make small changes to their equipment without having to submit an application for review each time. Moreover, the FDA will ensure that other aspects of the regulatory framework, such as new software verification tools, are flexible enough to meet the unique attributes of this rapidly evolving field and to ensure that these new technologies meet their safety and effectiveness standards. The main competitive landscape: Unicorn VS Enterprise Alliance In the field of digital therapy in the United States, the current major competitors are the unicorn company Proteus Digital Health and members of the Digital Therapy Alliance - Pear Therapeutics, Akili Interactive Labs, Propeller Health, WellDoc, Omada Health and Voluntis. Some digital therapy companies and products Source: Arterial Network As a unicorn company in the digital health arena, Proteus Digital Health specializes in digital healthcare with more than 450 patents. Its products include: drugs that support communication and communication after taking, wearable sensors for detecting drugs and capturing physiological responses, applications that support patient self-care and physician decision-making, and data analysis tools for doctors and health systems. In November 2017, Proteus' ABILIFY MYCITE system was approved by the FDA for New Drug Application (NDA), becoming the first approved drug-sensor combination for adults in the United States to track drug intake, treat schizophrenia and Major depression and so on. In October 2018, Proteus announced a further collaboration with Otsuka Pharmaceutical in digital therapies to apply technology to more mental health treatments to better meet the needs of patients with severe mental illness. Otsuka Pharmaceuticals paid Proteus $80 million in related equity and other payments and signed a five-year agreement to form a team to develop and commercialize the ABILIFY MYCITE system. The Digital Therapeutics Alliance was formed in October 2017 and includes Pear Therapeutics, Akili Interactive Labs, Propeller Health, WellDoc, Omada Health and Voluntis. Digital therapy company Akili Interactive Labs has raised a total of $68 million in 2018 with a total financing of more than $140 million. CLSA, Omidyar Technology Ventures, Digital Garage Group and Fearless Ventures participated in the investment. Akili provides digital treatment primarily through video games. For example, the AKL-T01 (also known as the "Evo ADHD Treatment Program") allows the user to control the virtual avatar by tilting the mobile device back and forth, in which the user must respond to the target by tapping the screen. Related applications can track user movements, monitor user behavior, and quickly adapt to the player. This sensory stimulation delivered through the game can act on specific neural circuits to treat cognitive dysfunction. In December 2017, Akili announced the results of the largest clinical trial of the AKL-T01. Currently, these trial data are being used for FDA approval for related digital therapies. Digital chronic disease management company Omada Health provides digital therapy for type 2 diabetes, heart disease and obesity. The Omada platform is an open system that allows users to customize their health needs by creating modular and personalized processes in their programs to address obesity-related diseases. Specifically, digital programs adjust their management recommendations based on various variables, including user statistics, morbidity, self-identification barriers, and medication adherence. In January of this year, Omada announced the launch of the largest clinical trial of digital diabetes prevention programs to date. In May, the project was fully accredited by the US Centers for Disease Control and Prevention (CDC). In September, Omada announced a partnership with Cigna. By January 2019, Omada's technology will integrate with Cigna's mobile products to provide digital healthcare services to more patients. Pear Therapeutics is a digital healthcare technology company that develops digital healthcare and drug/digital software portfolio solutions designed to improve patient outcomes and provide actionable data to guide clinicians in decision making. The company's leading product, ReSET, is the first FDA-approved prescription digital therapy that can be used as adjunctive therapy for drug abuse disorders such as stimulants, marijuana, cocaine or alcohol to help patients regain control of drug addiction. Other digital therapies include reSET-O for the treatment of opioid disability and reVIVE for the treatment of generalized anxiety and panic disorder. In March of this year, Novartis and Pear Therapeutics signed a partnership agreement to develop digital prescription therapy for patients with schizophrenia and multiple sclerosis (MS). This collaboration will combine Novartis's expertise in neurology, bed development and commercialization, as well as Pear Therapeutics' experience in digital prescription therapy design. Propeller Health, a provider of respiratory health management solutions, has a mobile platform that includes sensors, mobile applications, analytics and services to support respiratory health management, improve patient outcomes and reduce healthcare costs. Propeller's smart sensors continuously track patients' medications and record when and where they use the inhaler. In 2017, Propeller and Novartis Pharmaceuticals jointly developed a smart sensor customized for the Breezhaler series of chronic obstructive pulmonary disease (Ultibro Breezhaler, Onbrez Breezhaler and Seebri Breezhaler). In May of this year, Propeller Health partnered with Aptar Pharma to upgrade equipment and platforms for chronic respiratory diseases. Propeller claims that its products have received FDA 510K certification. WellDoc's main business is to provide diabetes management platforms for mobile phones and the cloud, and to work with insurance companies to provide diabetes management programs for patients. BlueStar is a one-stop digital health solution that compares patient data and sends analysis reports to medical teams to help patients better manage chronic conditions and achieve long-term health. In January of this year, WellDoc and Truven Health Analytics released a joint report that showed that BlueStar can save an average of more than $250 per patient per month. In July, WellDoc announced that it has expanded its digital diabetes management platform, BlueStar, to add weight management and hypertension testing. After that, the company will also launch a stand-alone product for high blood pressure. Voluntis' Insulia Diabetes Management System is a prescription-based medical device that provides insulin dose reminders and guidance to patients with type 2 diabetes based on blood glucose values ​​and other blood glucose-related data through smartphone applications. Insulia supports a wide range of treatment protocol configurations and evidence-based insulin adjustment rules for everyday clinical practice. Individualized treatment options are initially developed by medical professionals based on their individual circumstances, insulin prescriptions, and blood glucose goals. The dose adjustment algorithm is embedded in the application and no further validation by the medical professional is required after the initial setup of the system. Patient data is then automatically shared with the medical team, which can remotely monitor the patient's progress in achieving their treatment goals, thanks to customized reminders. In November 2016, the Insulia app was licensed by the FDA (510k) and the European CE mark. In November 2017, the solution was approved by the FDA to add two basic insulins – Basaglar and Tresiba – to the app. Prior to this, Insulia has been applied to insulin brands such as Lantus, Levemir and Toujeo. Development potential: as an auxiliary treatment, bring opportunities to pharmaceutical companies Reps Sangar of Ipsos Healthcare said that when explaining digital therapy to doctors, most people expressed interest, but more often as an adjunct to drugs, rather than a separate treatment. Specifically, when neuroscientists were asked about a virtual reality chronic disease product, only 7% said they would use it alone, 39% said they would use VR and drugs at the same time, and 43% said they would stick to it. Use drugs. For endocrinologists, when asked about a digital application for diabetes treatment, 22% said they would use a digital application and 27% would use an application with an insulin consultant, 38 % of people use traditional therapies. In addition, the pharmaceutical model itself can hinder the development of digital therapies, because in most countries, patents give pharmaceutical manufacturers 20 years or more of protection from the competition of generic drugs. But regulators can't provide the same protection for equipment or equipment. Competitors can only enter the market quickly by avoiding infringement of design patents and by incrementally changing the technology they use. Coupled with the huge differences in production and distribution, the development model of digital therapy is completely different from the drug distribution model. However, digital therapy can also offer pharmaceutical companies tremendous opportunities to change the way they develop or sell products. Proving the clinical and economic benefits of a product has always been a challenge for those seeking a product to enter the market. But digital therapies provide different perspectives on how drugs are being consumed, as well as real-time data on their impact. The accompanying sensors can tell the pharmaceutical company's response to their medications, affecting development, or signaling when relatively low levels of treatment are ineffective, demonstrating to doctors and payers that more effective treatment is needed. The enormous potential of digital therapy in the pharmaceutical industry has attracted many investors and partners in the industry. For example, Roche recently acquired mySugr for diabetes. In the long run, more pharmaceutical companies are likely to have their own digital therapeutic product line. But in the short term, most companies choose to work together, especially for digital devices that use data and patient participation to judge the effectiveness of the therapy, which can be used as an extended therapy. For example, pharmaceutical giant GlaxoSmithKline (GSK), in collaboration with digital therapist Propeller Health, has produced a "smart" inhaler for the treatment of asthma and chronic obstructive pulmonary disease. Both Peter Hames, CEO of Big Health, and Jorg Land, CEO of Sonormed, said that any evidence-based behavioral or mental health issues are suitable for digital therapy because about 80% of medical budgets are caused by chronic diseases, and a large part of them can be recognized. Knowing behavioral therapy is solved directly or indirectly. For example, there are only 4,500 neurologists in Germany, and an increase in population aging can lead to an increase in the number of patients. Some companies focus on early detection, but diagnosis only makes sense when it comes to treatment. Otherwise, patients may be negatively affected and the market will tend to offer solutions that have both therapeutic and preventive value. Edouard Gasser, CEO of Tilak Healthcare, believes that digital therapy can be applied to ophthalmology, neurology or psychiatry. For example, psychiatry is a university, because monitoring patients is critical, and therapist cannot be with the patient 24 hours a day. For people with depression, carry an application with you. More convenient. Although there is still a long way to go before digital therapy becomes mainstream therapy and solves important health problems. But as people become more aware of the need to set standards for safety and efficacy, digital innovators are also accelerating collaboration with payers, suppliers and pharmaceutical companies, and seeking faster regulatory approvals.
The fruit, corn, contains a lot of magnesium, which is known to be one of the few trace elements in the food and can help prevent cancer development.And the fruit corn contains a substance called glutathione, which can make some chemical carcinogens in the body toxic.To ward off cancer.
Although corn is highly nutritious, it is rich in crude fibre, so for children with delicate stomachs, too much of it can easily cause indigestion. If you add milk to the juice and then give it to your child, it will be less taxing on the stomach and more nutritious, and your child will be able to eat more food. (This method uses a wall breaker, but if you don't have one, a juicer is fine.)
If you would like to work with us, please send us a message on the website or contact us by email.
Delectable Sweet Corn Kernel,Sweet Corn Cob Kernels,Storing Fresh Corn Kernel,Baked Sweet Corn Kernels Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com