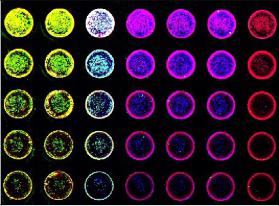
In cell western detection using the sapphire dual-mode multispectral laser imaging system
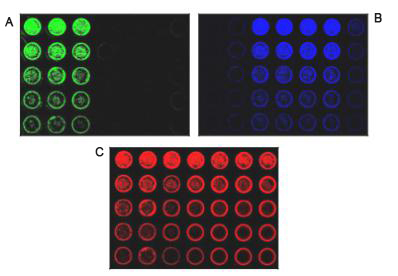
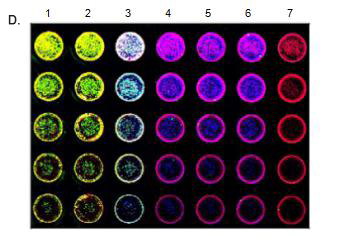
Introduction Western blotting was first proposed in 1979. Since then, improvements in technology, reagents, and imaging techniques have greatly expanded the use of Western blotting, making it one of the fundamental experiments in today's life sciences. However, the general workflow of Western blots has changed little. The protein is first extracted, then the protein is separated by electrophoresis, transferred and hybridized with primary and secondary antibodies, and finally developed. Through these steps, Western blotting requires a significant amount of time and expense on equipment, training, and reagents. The standard Western blotting process takes two days to get an image from the sample. Therefore, when analyzing a large number of samples or optimizing, it is easy to see that Western Blotting has a certain bottleneck. IN-cell western - Rethinking the standard approach by combining accurate quantification of proteins in Western blots with ELISA repeatability, rapidity and high throughput. The cells are grown in sterile culture wells and fixed in situ and permeabilized. Incubation with a specific primary antibody followed by incubation with a specific fluorescently labeled secondary antibody. The use of dyes in the near infrared (NIR) spectrum is commonly used to reduce the autofluorescence and noise associated with tissue culture plastic sheets. Figure 1: Typical in-cell western imaging The Azure Biosystems SapphireTM dual-mode multispectral laser imaging system is specifically designed to image multiple NIR channels (658 nm and 785 nm) and visible fluorescence wavelengths (488 nm and 520 nm) for multiple imaging in the well . This multiplex assay allows multiple proteins of interest to be evaluated in a single well. By using photomultiplier tubes (PMT) and avalanche photodiode (APD) detectors, Sapphire's signal selectivity, sensitivity and imaging speed make it an ideal choice for intracellular Western blotting. Materials and Method Cell culture HeLa cells were serially diluted and seeded into sterile 96-well tissue culture plates at a volume of 0.2 mL per well and grown to approximately 80%. All wells were permeabilized with 100% methanol for 15 minutes at room temperature. In-cell western After fixation and permeabilization, the cells were washed in PBS, blocked with PBS containing 1% fish gel for 1 hour at room temperature, and then a-tublin and beta-actin were incubated overnight at 4 °C. The samples were incubated with Azure Spectra 550 and Azure Spectra 800 for 60 minutes and the samples were washed three times with PBS. The second antibody RedDot TM 1 was incubated with nuclear staining for total cell number normalization. The sample plate was washed as previously described prior to imaging. Imaging After washing, imaging was performed using an Azure Sapphire dual-mode multispectral laser imaging system. Results and discussion In this description, we demonstrate the ability to quantify intracellular proteins using the Azure Biosystems SapphireTM dual-mode multispectral system. HeLa cells were incubated with a-tublin and beta-actin antibodies followed by Azure Spectra 550 and Azure Spectra 800 secondary antibody, respectively. All wells were RedDot TM 1 nuclear stain certain total cell number normalization. Images were acquired using the Azure Biosystems SapphireTM dual mode multispectral laser imaging system, as shown in Figure 2. The displayed image shows that higher sensitivity and specificity can be achieved. Serially diluted HeLa cells were seeded into 96-well plates, cultured, fixed, and permeabilized. A) Use the Azure Spectra 550 (green) to detect beta-Actin in columns 1-3. B) Use the Azure Spectra 800 (blue) to detect Tubulin in columns 3-6. C) The entire plate was stained with RedDot1 nuclear staining as a standardized control (red). D) Simultaneously scan each channel and then integrate its image using Sapphire Capture software. Western blotting of cells in 96-well plates allows accurate measurement of in-situ expression of intracellular proteins; and provides a high-throughput method to assess multiplex detection, endpoint detection, protein of interest, and reproducibility. By using NIR antibodies and the Azure SapphireTM dual-mode multispectral laser imaging system, the potential and throughput of multiple analyses is greatly enhanced. Green Prickly Ash,Pricklyash Peel Green Prickly Ash,Green Peppercorns Prickly Ash,Green Chinese Prickly Ash Sichuan Liuhang Agriculture Co.Ltd , https://www.lhagriculture.com