Rheumatoid arthritis new drug phase 3 clinically reaches all critical endpoints
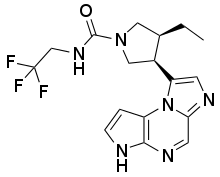
Rheumatoid arthritis new drug phase 3 clinically reaches all critical endpoints December 21, 2017 Source: WuXi PharmaTech Today, AbbVie announced that its phase 3 clinical trial SELECT-MONOTHERAPY has achieved positive results. The trial evaluated the efficacy of the JAK1 inhibitor upadacitinib (ABT-494) as a monotherapy for patients with moderate to severe rheumatoid arthritis (RA) who did not respond adequately to methotrexate treatment. Rheumatoid arthritis affects 23.7 million people worldwide and is a chronic debilitating disease. Methotrexate is commonly used as a first-line treatment for RA, but many patients are unresponsive or intolerant to methotrexate, which puts them at risk for disease progression. These patients are in urgent need of new treatments to alleviate the disease. The upadacitinib developed by Abbott is a potential oral drug for research. It selectively inhibits JAK1, which plays an important role in the pathogenesis of RA and other immune-mediated inflammatory diseases. Currently, Phase 3 clinical trials for the treatment of psoriatic arthritis with upadacitinib are underway and are being studied for the treatment of Crohn's disease, ulcerative colitis, ankylosing spondylitis and atopic dermatitis. ▲The molecular structure of Upadacitinib (Source: Wikipedia) The efficacy of Upadacitinib has also been confirmed in clinical trials. SELECT-MONOTHERAPY is a phase 3 multicenter, randomized, double-blind, parallel-group study designed to evaluate the safety and efficacy of upadacitinib in the treatment of moderate to severe RA patients with stable doses of methotrexate Insufficient response. Subjects were randomized from methotrexate to upadacitinib monotherapy (15 mg or 30 mg once daily) or blinded to a stable dose of methotrexate. The primary endpoint of the first phase included the proportion of patients achieving ACR20 response* and low disease activity (LDA)** after 14 weeks of treatment. Secondary endpoints included the proportion of patients who achieved ACR50, ACR70, and clinical remission at week 14. The second phase was a long-term extension of the blinded trial, evaluating the long-term safety, tolerability, and efficacy of upadacitinib monotherapy in two doses (15 mg and 30 mg) once in the first phase. *ACR20/50/70 is defined by the American College of Rheumatology as 20%/50%/70% joint swelling improvement, plus the following 3 items: patients assess pain, systemic disease activity and body function, doctors' disease activity and acute phase reactants Comprehensive assessment ** LDA is defined as having a clinical response disease activity score of 28 joint counts (C-reactive protein) (DAS28 [CRP]) less than or equal to 3.2 ▲Upadacitinib is expected to treat a variety of immune-related diseases (Source: Aibowei official website) The results showed that 68%/42%/23% of patients who switched to 15 mg once daily of upadacitinib achieved ACR20/50/70 after 14 weeks of treatment, and 71 of the patients who switched to 30 mg once daily upadacitinib %/52%/33% of patients achieved ACR20/50/70, and 41%/15%/3% of patients receiving methotrexate achieved this result. In addition, at week 14, 45% and 53% of patients in the 15 mg and 30 mg dose groups achieved LDA, respectively, compared with 19% in the methotrexate group and 28% and 41% in the 15 mg and 30 mg dose groups, respectively. The patient achieved clinical remission, compared with 8% in the methotrexate group. Therefore, both doses of upadacitinib monotherapy reached the primary end point of ACR20 and LDA, as well as all critical secondary endpoints. “The positive results of SELECT-MONOTHERAPY are encouraging because they support upadacitinib for the first time as a potential treatment for ketamine without treatment,†said Dr. Michael Severino, Executive Vice President and Chief Scientific Officer of Abbott’s R&D department: "These findings and increasing data suggest that upadacitinib may be an effective treatment option for patients with rheumatoid arthritis. We look forward to sharing the upadacitinib phase 3 rheumatoid arthritis project with the scientific community in 2018. Other data." "This trial addresses the clinical impact of the conversion of methotrexate to upadacitinib in patients who are underreactive to methotrexate. The results suggest that both doses of upadacitinib can provide a clinically meaningful response," Vienna Medical University, Rheumatology Dr. Josef S. Smolen, an immunologist and one of the researchers at the trial, said: "These findings support the use of upadacitinib monotherapy as a treatment option for patients with rheumatoid arthritis." We expect this drug to continue to perform well and bring new treatment options to patients. Reference materials: [1] AbbVie Scores Key Trial Win for RA Drug With One Fatal Event Reported [2] AbbVie official website Lactic Acid,Biodegradable Lactic Acid,Lactic Acid Liquid,Poly Lactic Acid SHANDONG BAISHENG BIOTECHNOLOGY CO., LTD , https://www.baishengbioproducts.com